this post was submitted on 12 Jan 2025
196 points (90.2% liked)
Science Memes
11604 readers
1112 users here now
Welcome to c/science_memes @ Mander.xyz!
A place for majestic STEMLORD peacocking, as well as memes about the realities of working in a lab.

Rules
- Don't throw mud. Behave like an intellectual and remember the human.
- Keep it rooted (on topic).
- No spam.
- Infographics welcome, get schooled.
This is a science community. We use the Dawkins definition of meme.
Research Committee
Other Mander Communities
Science and Research
Biology and Life Sciences
- !abiogenesis@mander.xyz
- !animal-behavior@mander.xyz
- !anthropology@mander.xyz
- !arachnology@mander.xyz
- !balconygardening@slrpnk.net
- !biodiversity@mander.xyz
- !biology@mander.xyz
- !biophysics@mander.xyz
- !botany@mander.xyz
- !ecology@mander.xyz
- !entomology@mander.xyz
- !fermentation@mander.xyz
- !herpetology@mander.xyz
- !houseplants@mander.xyz
- !medicine@mander.xyz
- !microscopy@mander.xyz
- !mycology@mander.xyz
- !nudibranchs@mander.xyz
- !nutrition@mander.xyz
- !palaeoecology@mander.xyz
- !palaeontology@mander.xyz
- !photosynthesis@mander.xyz
- !plantid@mander.xyz
- !plants@mander.xyz
- !reptiles and amphibians@mander.xyz
Physical Sciences
- !astronomy@mander.xyz
- !chemistry@mander.xyz
- !earthscience@mander.xyz
- !geography@mander.xyz
- !geospatial@mander.xyz
- !nuclear@mander.xyz
- !physics@mander.xyz
- !quantum-computing@mander.xyz
- !spectroscopy@mander.xyz
Humanities and Social Sciences
Practical and Applied Sciences
- !exercise-and sports-science@mander.xyz
- !gardening@mander.xyz
- !self sufficiency@mander.xyz
- !soilscience@slrpnk.net
- !terrariums@mander.xyz
- !timelapse@mander.xyz
Memes
Miscellaneous
founded 2 years ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
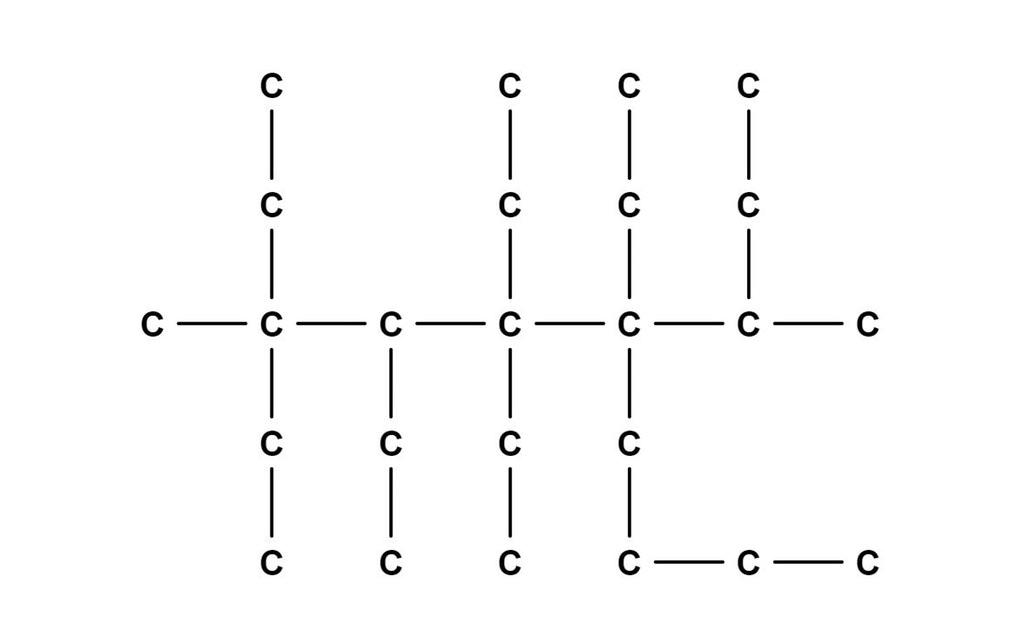
Fixed the charge on your 3-methyl-3,4,5,5,6-pentaethyl-6-butan-2-yl decane ion, aka lossane.
(It's been a while since I last did chemistry, so apologies if I messed up the nomenclature a bit)
As someone who paid enough attention in highschool chemistry to get a B, and occasionally watches Nile(red/blue) and E&I videos.... I know some of these words/symbols!
actually we start numbering by minimising the number of highest order addition, which is the isobutyl, if it gets same number regardless, then we try to minimise the sum of numbers, so i think it should be called
5-(isobutyl)-5,6,6,7,8-penta-ethyl-8-methyl-decane (I am assuming hydrogen's are present, just not represented, because that usually is the case)
I may alo be wrong here, it has been 4 years since I have been required to do nomenclature myself
Ah, my chemistry department taught me the other way around. I distinctly remember the phrase "methyl-ethyl" being thrown around a bit. Additionaly, we were taught to be more specific about isomers, hence me using butan-2-yl instead of isobutyl.
Then again, scientists often disagree strongly about things like this, so we could both be right. Also, there's a good chance this is just the A-Level specification being weird. I left my old textbook at my student flat, though, so I won't be able to check for a couple of weeks.
I could also be downright wrong myself.
As for the hydrogens, I had assumed they were not present, and that this lossane molecule is an ion with a charge of -50. This is borderline impossible to achieve in real life, of course.
Now i remember we also had to specify what isobutyl (technically just 1 isobutyl exists (not counting stereoisomers), and other form would be tertbutyl). But giving highest priority and minimising sum were definitely something we were taught.
I was free enough to look it up this time - IUPAC guidelines for organic chem - https://iupac.org/wp-content/uploads/2021/06/Organic-Brief-Guide-brochure_v1.1_June2021.pdf (or more generally https://iupac.org/what-we-do/nomenclature/brief-guides/)
Section 7 ( c ) Lowest locant(s) for principal characteristic group(s)
Although I also remember just as we completed our unit on nomenclature, all we got was "common names", now i was supposed to know of the top of my head what a cumene is (which I think is isopropyl benzene (not going to check this one)). Same thing happened with polymers, we were taught IUPAC, and then again, "industrial names"
Any idea what a molecule like that would be useful for?
Fuel? Hydrocarbons like that are quite combustible. It could also be incorporated into a lipid or something.